The progression of myopia is believed to involve a cascade of signals originating in the retina, which are transmitted to the retinal pigment epithelium (RPE) and choroid, ultimately leading to physical changes in the eyeball driven by scleral remodeling. Recent findings also highlight the eye's direct connection to the brain via neural pathways and its systemic linkage to other organs through blood flow entering the choroid.
Our research aims to elucidate both local interactions among the retina, RPE, choroid, and sclera, as well as systemic connections between the eye, brain, and intestinal tract. By unraveling this intricate network of inter-organ and inter-tissue communication, we seek to deepen our understanding of how these interactions contribute to ocular morphology and function.
Hypoxic Response A Novel Approach to the Treatment of Intractable Retinal Diseases
At the Laboratory of Photobiology in the Department of Ophthalmology, Keio University School of Medicine, we are exploring how disruptions in oxygen homeostasis contribute to the onset and progression of retinal diseases. The retina is one of the most oxygen-demanding tissues in the body, and even subtle fluctuations in its microenvironmental oxygen levels are closely associated with the pathogenesis of intractable retinal disorders such as age-related macular degeneration, diabetic retinopathy, and glaucoma.
Our laboratory has revealed that hypoxia-inducible factors (HIFs)—transcription factors that regulate cellular responses to hypoxic stress—play pivotal roles in both the initiation and progression of these diseases. Through a multifaceted approach employing various disease models, we have systematically elucidated the involvement of HIFs in retinal pathology.
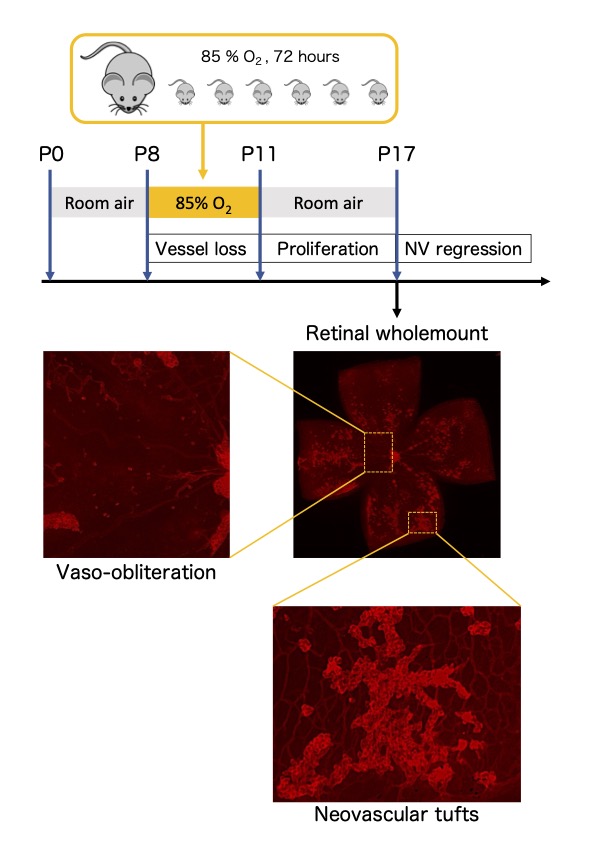
Specifically, we have utilized mouse models that recapitulate key clinical conditions: laser-induced choroidal neovascularization for age-related macular degeneration, oxygen-induced retinopathy for diabetic retinopathy and retinopathy of prematurity, and light-induced retinal damage for retinitis pigmentosa. In these models, we observed pathological overexpression of HIFs during disease development and demonstrated that pharmacologic or genetic inhibition of HIFs suppresses disease progression. Furthermore, studies using genetically engineered mouse models further support the pivotal role of HIFs in disease pathogenesis. Complementing these in vivo findings, mechanistic analyses using various retinal cell lines have allowed us to identify disease-specific HIF-regulated pathogenic factors.
Based on these findings, the Laboratory of Photobiology is actively pursuing translational research, aiming to establish novel therapeutic strategies for retinal diseases through the perspective of oxygen metabolism.
Visual Restoration Research Creating Sight Again Through Light
We are conducting basic research on visual restoration using "optogenetics" as a novel approach to treat visual impairments caused by retinal degenerative diseases.
Optogenetics involves introducing light-sensitive proteins into retinal neurons that no longer respond to light, thereby artificially enabling them to react to light stimuli. Even in the late stages of retinal degeneration, where the photoreceptors responsible for vision have been lost, many of the inner retinal neurons—such as bipolar cells and retinal ganglion cells—remain viable. By conferring light sensitivity to these surviving cells, it becomes possible to utilize the remaining neural circuits to deliver visual information to the brain once again.
Our laboratory is actively developing and characterizing a wide variety of light-sensitive proteins—including microbial, animal-derived, and chimeric types—by comparing their light sensitivity, response speed, and signal amplification properties to identify the most suitable molecules for visual restoration.
A particular focus has been placed on the development and functional analysis of the chimeric opsin “GHCR (Gloeobacter and human chimeric rhodopsin)” which demonstrates high sensitivity and rapid response even under low-light conditions. In mouse models, GHCR has been shown to improve visually guided behavior and offer neuroprotective effects, contributing to its successful translation into clinical application.
We are also exploring which retinal cells should be targeted for gene delivery to achieve more physiological and sensitive visual signal transmission. These studies include experiments targeting retinal ganglion cells, bipolar cells, and even amacrine cells.
By reconstructing the function of neural circuits using light, our laboratory aims to restore lost vision. Through continued foundational research, the Photobiology Laboratory is paving the way for the next generation of vision restoration therapies.

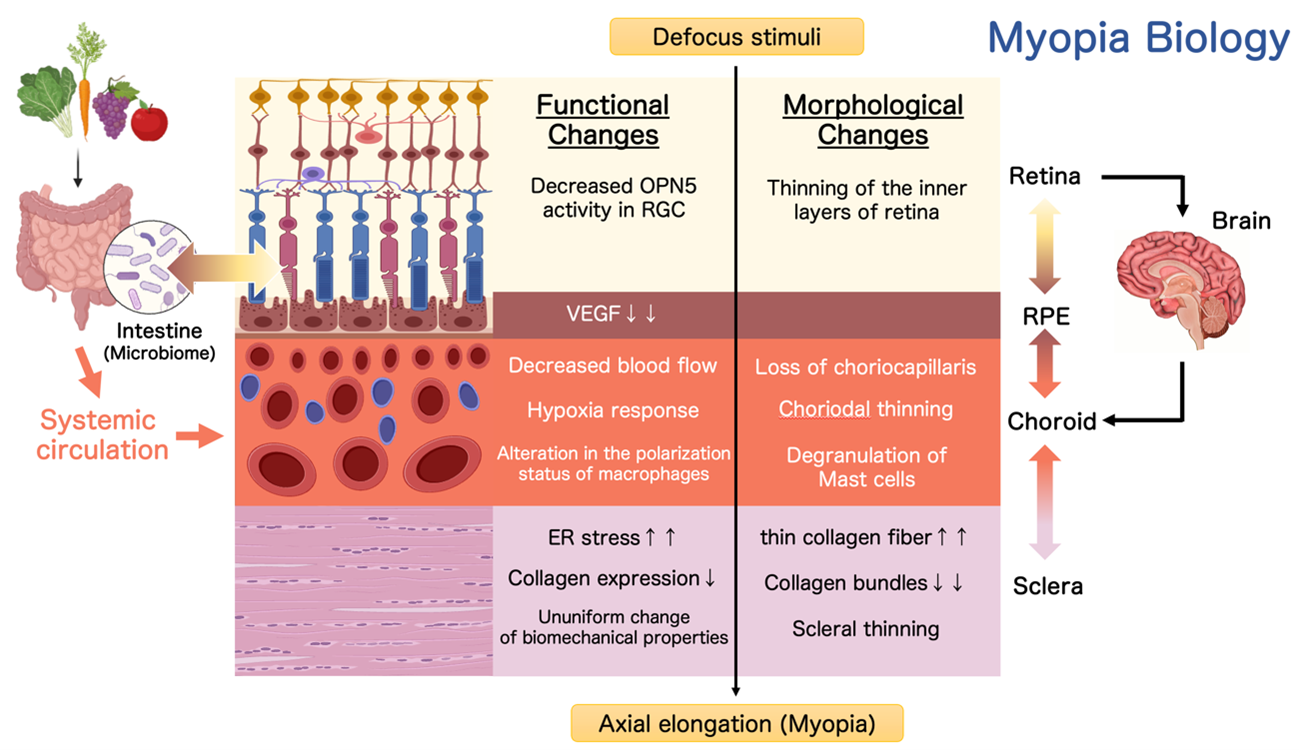
Myopia Biology Decoding, Understanding, and Conquering Myopia
Myopia is a refractive abnormality resulting from excessive elongation of the eyeball along its anteroposterior axis (axial elongation). This acquired morphological change significantly increases the risk of vision-threatening conditions, including glaucoma, retinal detachment, and macular degeneration. Epidemiological data from our study revealed that about 95% of a junior high school students in Tokyo are myopic, highlighting the alarming global rise in myopia prevalence. As myopia is now recognized as a major risk factor for visual impairment, there is an urgent need to develop effective intervention strategies.
In our laboratory, we are dedicated to pioneering a new research field, "Myopia Biology," which seeks to develop effective intervention strategies for myopia by elucidating the molecular and cellular biological mechanisms underlying the acquired morphological changes in the eyeball associated with this condition. We are conducting the following research, focusing mainly on the posterior eye (retina, retinal pigment epithelium, choroid, sclera).
-
1. The Role of Non-Visual Opsin in Myopia Development and Suppression
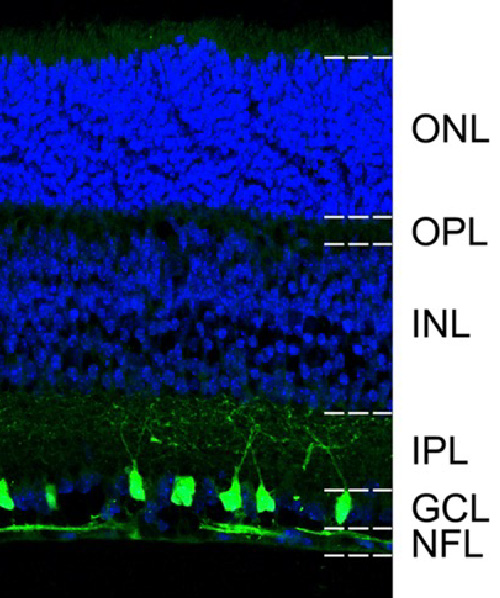
Unlike rhodopsin, non-visual opsins are involved in light-dependent physiological processes beyond vision, such as circadian rhythm regulation and thermoregulation. Our research has identified OPN5 (neuropsin), a non-visual opsin expressed in retinal ganglion cells (RGCs), as a key factor in suppressing myopia. Specifically, OPN5 mediates this suppression by detecting violet light within the wavelength range of 360–400 nm. Utilizing advanced neuroscience and genetic engineering techniques, we are investigating the mechanisms by which OPN5 in RGCs regulates eyeball growth and prevents myopia progression.
-
2. Choroidal Immunology
The choroid is a vascular and pigmented tissue situated between the retina and sclera. Growing evidence indicates that alterations in choroidal thickness, driven by factors such as diminished blood flow and vascular degeneration, may contribute to the development of myopia. Additionally, the choroid exhibits the highest blood flow per unit volume of any tissue in the body. Its constant exposure to systemic circulation via blood flow also positions it as a unique immune niche within the eye, housing a dense population of resident immune cells.
Our research investigates the physiological roles and pathological implications of these choroidal immune cells, with a focus on their interactions with neural and vascular networks. By elucidating these mechanisms, we aim to uncover novel pathways linking immune activity to ocular growth regulation and myopia pathogenesis.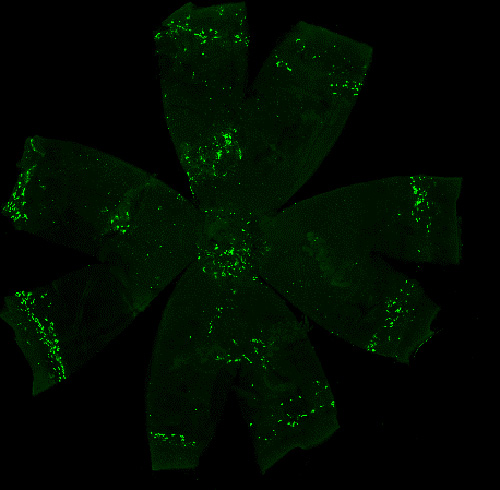
-
3. Molecular Mechanisms of Choroidal-Scleral Remodeling and Regional Heterogeneity
The sclera, primarily composed of collagen and a small number of fibroblasts, along with the choroid located adjacent to it, provides rigidity and elasticity essential for maintaining the shape of the eyeball. Remodeling of these tissues plays a critical role in facilitating changes in eyeball shape.
Our research has identified that the PERK and ATF6 pathways—downstream signaling pathways activated by endoplasmic reticulum (ER) stress in scleral fibroblasts—regulate scleral remodeling during myopia progression. Based on these findings, we are working to develop myopia-inhibiting drugs targeting ER stress.
Furthermore, since eyeball deformation associated with myopia is not uniform but is particularly pronounced along the anteroposterior axis, it is likely that cellular and molecular changes driving this process are regionally specific. To address this, we are conducting studies aimed at elucidating the molecular biological and mechanical changes that occur in the myopic eye.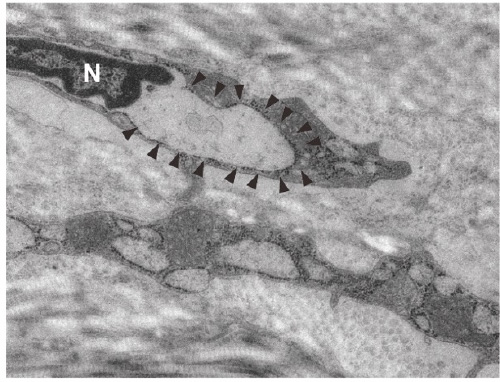
-
4. Morphological and Functional Regulation of the Eye Through Inter-organ and inter-tissue Interactions
Bridging Vision Restoration Research Challenges Toward Clinical Application
Realizing vision restoration requires not only innovative basic research but also effective translational efforts that bridge laboratory discoveries to clinical applications. Particularly in the field of gene therapy, significant hurdles exist, including the establishment of reliable visual function assessment methods and the validation of treatment efficacy through clinical trials.
At the Laboratory of Photobiology, Keio University, we are actively engaged in translational research efforts to advance vision restoration technologies into societal implementation. In collaboration with Restore Vision, a spin-off venture from our laboratory, we are establishing and validating clinical evaluation systems critical for successful translation.
For instance, we are collaboratively developing a comprehensive multi-dimensional visual assessment framework designed to accurately evaluate treatment outcomes in patients with ultra-low vision. This framework integrates retinal light sensitivity tests (Full-field Stimulus Testing; FST), behavioral tasks (such as Table Test and Door Task), and quality-of-life assessments (Impact of Vision Impairment – Very Low Vision; IVI-VLV). This approach is beginning to enable scientific detection of subtle visual changes that traditional visual acuity charts fail to capture.
Furthermore, our partnership with Restore Vision extends to conducting clinical trials to verify gene therapy products' safety and efficacy. Together, we address medical and technical aspects necessary for rigorous clinical evaluation. By systematically overcoming various challenges, from establishing robust evaluation methods to conducting clinical validations, we strive to make vision restoration therapies tangible.
By integrating fundamental molecular research with practical clinical applications, we aspire to provide new possibilities for individuals who have lost their vision. The Laboratory of Photobiology remains dedicated to advancing translational research, with a clear vision of translating our scientific achievements into practical medical benefits.


Low Vision Support Initiatives
Our laboratory is dedicated to improving the quality of life for individuals with visual impairments or those experiencing difficulties with vision. We provide support through our Low Vision Outpatient Clinic and the Low Vision Care Hub (LVCH).
-
1.Low Vision Outpatient Clinic
The Low Vision Outpatient Clinic is a specialized medical service that provides support to individuals experiencing difficulties in daily life due to decreased visual acuity or reduced visual fields. Our clinic is available not only to those who meet the criteria for a disability certificate but also to individuals facing various visual challenges, such as “difficulty seeing clearly even with glasses or contact lenses,” “extreme sensitivity to glare,” or “difficulty reading small print.”
At the clinic, we conduct a thorough assessment of visual function and provide individualized support based on each patient's living environment and specific needs. Our services include prescribing tinted or corrective glasses, selecting assistive devices such as magnifiers and electronic reading aids, advising on glare reduction measures, referring patients to white cane training and daily living skills programs, and introducing the use of electronic devices for information accessibility.
The primary goal of the Low Vision Outpatient Clinic is to “help individuals with visual impairments maintain their independence and quality of life.” We strive to support patients in improving their daily lives and empowering them to participate in social activities with confidence.
For appointments, please contact us in advance to schedule a consultation. -
2.Low Vision Care Hub (LVCH)
The Low Vision Care Hub (LVCH) is an in-hospital outreach space jointly operated by Keio University and the Japan Guide Dog Association.
LVCH serves as a hub to connect individuals with visual difficulties to appropriate support services, including medical care, welfare, education, and employment. By collaborating with various organizations and institutions, we provide accurate information and referrals. Our consultations are primarily handled by trained staff who have visual impairments themselves, ensuring a supportive and informed environment. The hub is open not only to patients but also to their families, friends, and professionals from related organizations.
No appointment or referral is required, and individuals who are not patients of Keio University Hospital are also welcome to seek assistance.
For more details, please visit the Low Vision Care Hub website via the link below.



